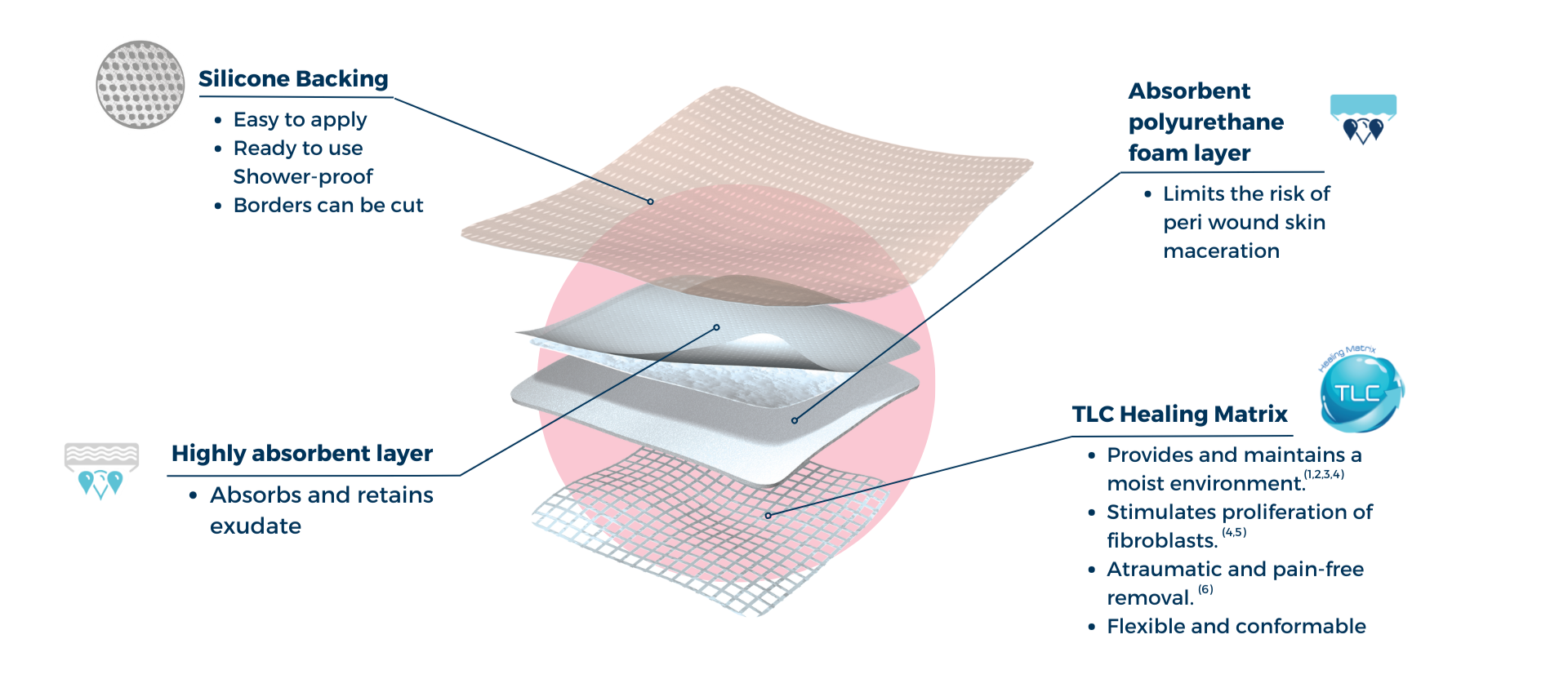
UrgoTul Absorb Border is an adhesive foam dressing with the Technology Lipido Colloid (TLC) matrix.
In contact with wound exudates, the hydrocolloid particles gel and interact with the petrolatum component of UrgoTul Absorb border to form a lipido-colloid gel in contact with the wound, providing and maintaining a moist environment that promotes the healing process.(1,2,3,4) UrgoTul Absorb Border has pro-healing efficacy, stimulating the proliferation of fibroblasts – enabling the key cells for healing.(4,5)
The benefits of TLC
- Provides and maintains a moist environment that promotes healing.(1,2,3,4)
- Atraumatic removal that doesn’t damage any newly formed granulation tissue.(6)
- Provides pain-free dressing changes for the patient.(6)
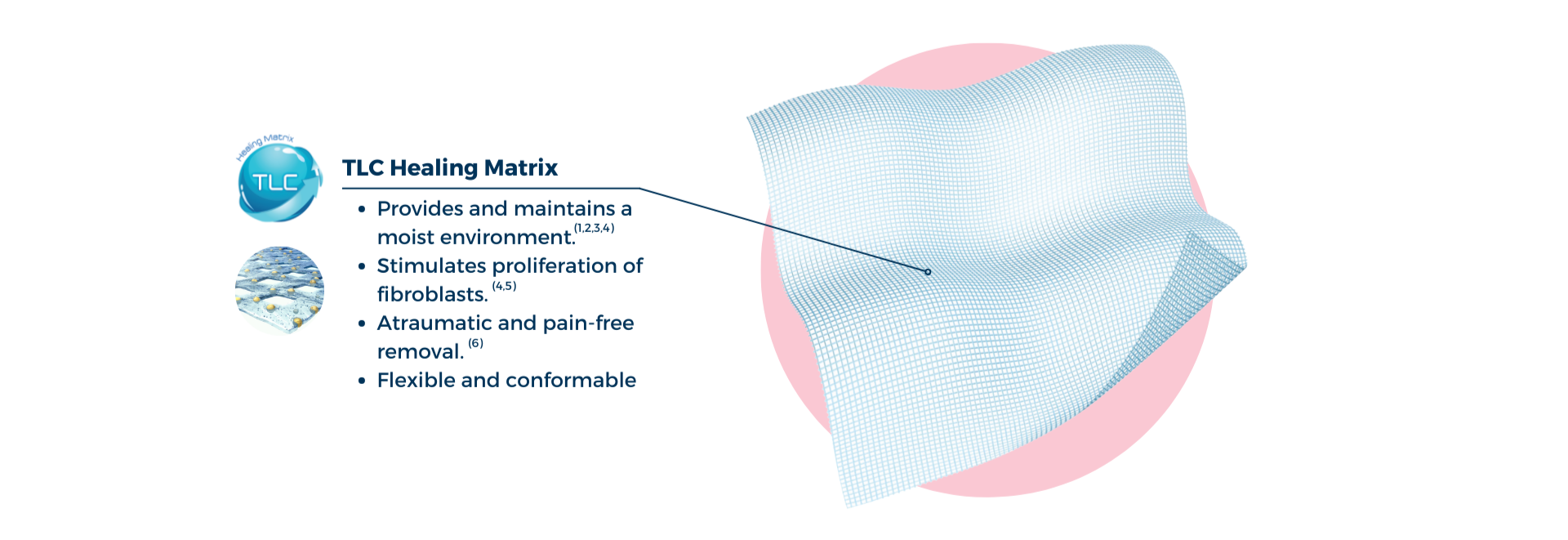
UrgoTul is a non-occlusive flexible and conformable dressing comprising of a polyester mesh impregnated with Technology Lipido-Colloid (TLC) healing matrix.
In contact with wound exudates, the hydrocolloid particles gel and interact with the petrolatum component of UrgoTul to form a lipido-colloid gel in contact with the wound, providing and maintaining a moist environment that promotes the healing process.(1,2,3,4) UrgoTul has pro-healing efficacy, stimulating the proliferation of fibroblasts – enabling the key cells for healing.(4,5)
The benefits of TLC:
- Provides and maintains a moist environment that promotes healing.(1,2,3,4)
- Atraumatic removal that doesn’t damage any newly formed granulation tissue.(6)
- Provides pain-free dressing changes for the patient.(6)
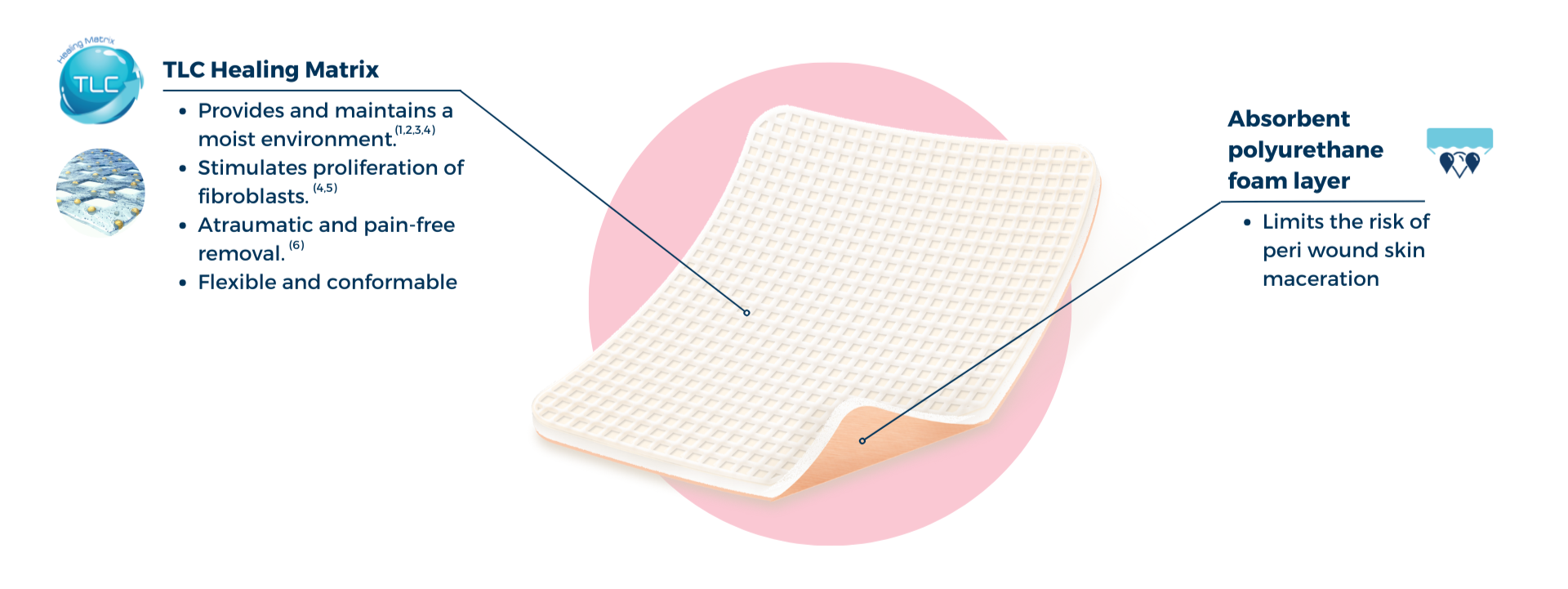
UrgoTul Absorb is an non-adhesive foam dressing with the Technology Lipido Colloid (TLC) matrix.
In contact with wound exudates, the hydrocolloid particles gel and interact with the petrolatum component of UrgoTul Absorb to form a lipido-colloid gel in contact with the wound, providing and maintaining a moist environment that promotes the healing process.(1,2,3,4) UrgoTul Absorb has pro-healing efficacy, stimulating the proliferation of fibroblasts – enabling the key cells for healing.(4,5)
The benefits of TLC:
- Provides and maintains a moist environment that promotes healing.(1,2,3,4)
- Atraumatic removal that doesn’t damage any newly formed granulation tissue.(6)
- Provides pain-free dressing changes for the patient.(6)
UrgoTul Absorb Border is indicated for the treatment of:
- Acute wounds (such as second degree burns, dermabrasions, traumatic wounds, postoperative wounds….) and chronic wounds (such as leg ulcers, pressure ulcers, diabetic foot ulcers) at the granulation and epithelialisation stage
- The sacrum version is recommended for exuding wounds located in the sacral area (sacral pressure ulcers….).
Contra-indications:
Known sensitivity to the dressing.
UrgoTul is indicated for the management of:
- Acute wounds (burns, traumatic wounds, abrasions, post-operative wounds) and chronic wounds (leg ulcers, pressure ulcers and diabetic foot ulcers) at the granulation and epithelialisation stage.
- Epidermolysis bullosa (EB).
Contra-indications:
Known sensitivity to the dressing.
UrgoTul Absorb is indicated for all exuding chronic wounds (leg ulcers, pressure ulcers, diabetic foot ulcers), acute wounds (2nd degree burns, skin abrasions, traumatic wounds, post-operative wounds….) and for the local treatment of fungating wounds in combination with a specific treatment.
UrgoTul Absorb Heel is indicated for all exuding wounds located in the heel area, chronic wounds (heel pressure ulcers….) and acute wounds (2nd degree burns, skin abrasions, traumatic wounds, post-operative wounds….).
Due to the non-adhesive nature of the dressing, Urgotul Absorb is recommended in the treatment of wounds in which the surrounding skin is weakened.
Contra-indications:
Known sensitivity to the dressing.
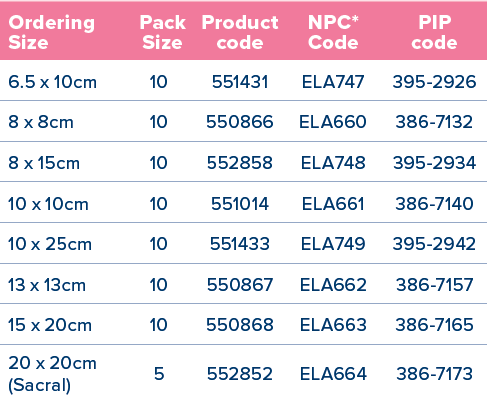
*National Product Code for NHS Supply Chain
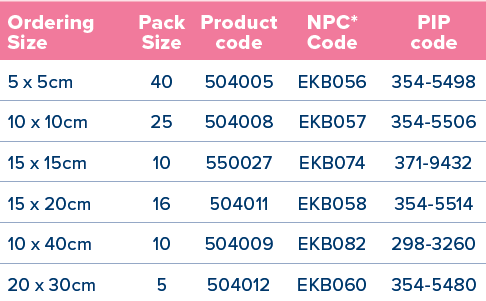
*National Product Code for NHS Supply Chain
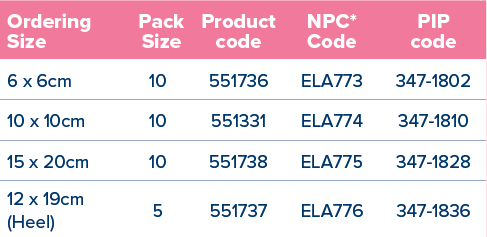
*National Product Code for NHS Supply Chain
Preparation of the wound:
- Clean the wound as per local protocol.
- If an antiseptic is first used, carefully rinse the wound with normal sterile saline before applying UrgoTul Absorb Border.
- Dry the surrounding skin carefully.
Application of the dressing:
- Remove the protective wings.
- Apply the soft-adherent side of UrgoTul Absorb Border to the wound (the adhesive silicone border should be at least 1 cm away from the wound).
- Smooth the dressing over the wound.
- Apply compression bandaging over the dressing when prescribed.
Application of the sacrum version:
- Place the dressing with the tip facing the sacral area.
Removal of the dressing:
- Press down on the healthy skin, lift on the corner of the dressing and remove it carefully.
Dressing changes:
UrgoTul Absorb Border dressing may be changed every 2 to 4 days, and left in place up to 7 days depending on the level of exudate and the condition of the wound.
Precautions for use:
- As it includes a super-absorbent layer, the central pad should not be cut. However, the adhesive edges can be cut if necessary using sterile scissors to fit the dressing to different wound shapes.
- If clinical signs of local infection are noted, treatment can be changed to an antibacterial dressing from the Urgo range, dependent on clinical judgement.
- Any excess hair should be cut close to the skin to ensure good contact with the wound.
- In case of concomitant use with cream, an ointment, an emulsion, let the skin dry before the dressing application.
- Urgotul Absorb Border must not be used in a hyperbaric chamber.
- Single use sterile individual packaging: re-using a single use dressing may lead to risks of infection.
- Check that the sterile protector is intact before use. Do not use if package is damaged.
- Do not re-sterilise the dressing.
Preparation of the wound:
- Prepare the wound according to local protocol. If an antiseptic is first used, rinse the wound thoroughly with saline solution before applying UrgoTul.
Application of the dressing:
- Remove the protective transparent film.
- Apply UrgoTul to the wound in a single layer.
- UrgoTul can be cut with sterile equipment to fit the size of the wound and the surrounding skin.
- Cover UrgoTul with a secondary dressing suitable to absorb the level of wound exudate.
- Hold secondary dressing in place with a conforming bandage, adhesive tape or an elasticated tubular bandage.
Dressing changes:
- UrgoTul should be changed every 2 to 4 days depending on the type of wound and its clinical condition. UrgoTul may be left in place for up to 7 days dependent on wound condition and protocol (when prescribed with a compression bandage system for venous leg ulcers).
- For patients with epidermolysis bullosa, the dressing should be changed after 1 to 3 days.
Precautions for use:
- UrgoTul adheres to latex surgical gloves. It is therefore recommended that gloves be moistened with saline in order to make the handling of UrgoTul
- If clinical signs of local infection are noted, treatment can be changed to an antibacterial dressing dependent on clinical judgement.
- In the case of deep wounds and fistula wounds, a section of the dressing should be left visible to enable easy removal.
- UrgoTul must not be used in a hyperbaric chamber.
- Single use sterile individual packaging: re-using a single use dressing may lead to risks of infection.
- Check that the sterile protector is intact before use. Do not use if package is damaged.
- Do not re-sterilize the dressing.
Preparation of the wound:
Standard and heel versions:
- Clean the wound as per local protocol, and then rinse the wound with normal sterile saline.
- If an antiseptic has been used previously, carefully rinse the wound with normal saline before applying UrgoTul Absorb.
- Dry the surrounding skin carefully.
Application of the dressing:
- If required UrgoTul Absorb and UrgoTul Absorb Heel can be cut using sterile equipment to adjust the size of the dressing to fit the wound.
Standard version:
- Remove the protective wings.
- Apply the soft-adherent side of UrgoTul Absorb to the wound.
- Secure the dressing in place with a suitable bandage.
- Apply compression bandaging over the dressing when prescribed.
Heel version:
- Remove the protective wings and place the heel of the foot in the centre of the narrow
- part of the arrow (shaped dressing (soft-adherent TLC side), pointing in the same direction.
- Fold the back of the dressing up and around the back of the heel.
- Mould the edges up and around the foot.
- Secure the dressing in place with a suitable bandage.
Dressing changes:
- UrgoTul Absorb and UrgoTul Absorb Heel dressings should be changed every 2 to 4 days, depending on the exudate volume and the clinical condition of the wound.
- When prescribed with a compression bandage system for venous leg ulcers, UrgoTul Absorb and UrgoTul Absorb Heel may be left in place for up to 7 days dependent on wound condition and local protocol.
Precautions for use:
- The soft-adherent TLC layer of UrgoTul Absorb adheres to surgical gloves (latex). It is therefore recommended to avoid contact with the soft-adherent TLC layer.
- If clinical signs of local infection are noted, treatment can be changed to an antibacterial dressing, dependent on clinical judgement.
- UrgoTul Absorb must not be used in a hyperbaric chamber.
- Single use sterile individual packaging: re-using a single use dressing may lead to risks of infection.
- Check that the sterile protector is intact before use. Do not use if package is damaged.
- Do not re-sterilise.
Get in touch with your local representative for more information about the UrgoTul Range
References:
1. Le Berre M, Lurton Y, Maia S, Roebroeck V, Durand J, Gicquel V, Basle B. Pansements impregnes : tulles/interfaces. Poster, CPC 2005, Paris.
2. Parpex P. et al. Management of venous leg ulcers with Cellosorb Micro-adherent dressing: results of a multi-centre clinical trial. Phlebologie 2010; 63: 76-82.
3. Meaume S, et al. Use of a new, flexible lipidocolloid dressing on acute and chronic wounds: results of a clinical study. J Wound Care. 2011;20(4):180,182-5.
4. Bernard., F.X., Barrault, C., Juchaux, F., et al.Stimulation of the proliferation of human dermal fibroblasts in vitro by a lipidocolloid dressing. J Wound Care 2005; 14: 5, 215–220. (Study conducted on UrgoTul)
5. FX. Bernard, F. Juchaux et al., Effets d’un pansement lipido colloide sur la production de matrice extracellulaire. Journal des Plaies et gCicatrisations, 2007. (Study conducted on Urgotul).
6. Meaume, S., Teot, L., Lazareth, I. et al. The importance of pain reduction through dressing selection in routine wound management: the MAPP study. J Wound Care 2004; 13: 10, 409–413.

