A LOCAL TREATMENT FOR YOUR PATIENTS AT RISK OF DELAYED HEALING
With its unique TLC-NOSF* Healing Matrix** and its poly-absorbent fibres, the UrgoStart Plus treatment range is the only local treatment proven to close wounds sooner by addressing two key local factors:
- Continuously cleans: its poly-absorbent fibres trap and retain exudate, slough and debris.(1,2)
- Heals & closes the wound sooner: its TLC-NOSF** healing matrix reduces excess matrix metalloproteinases (MMPs), rebalancing the wound and closing it sooner.(2,3,4)

EFFECTIVE IN ALL HEALING PHASES

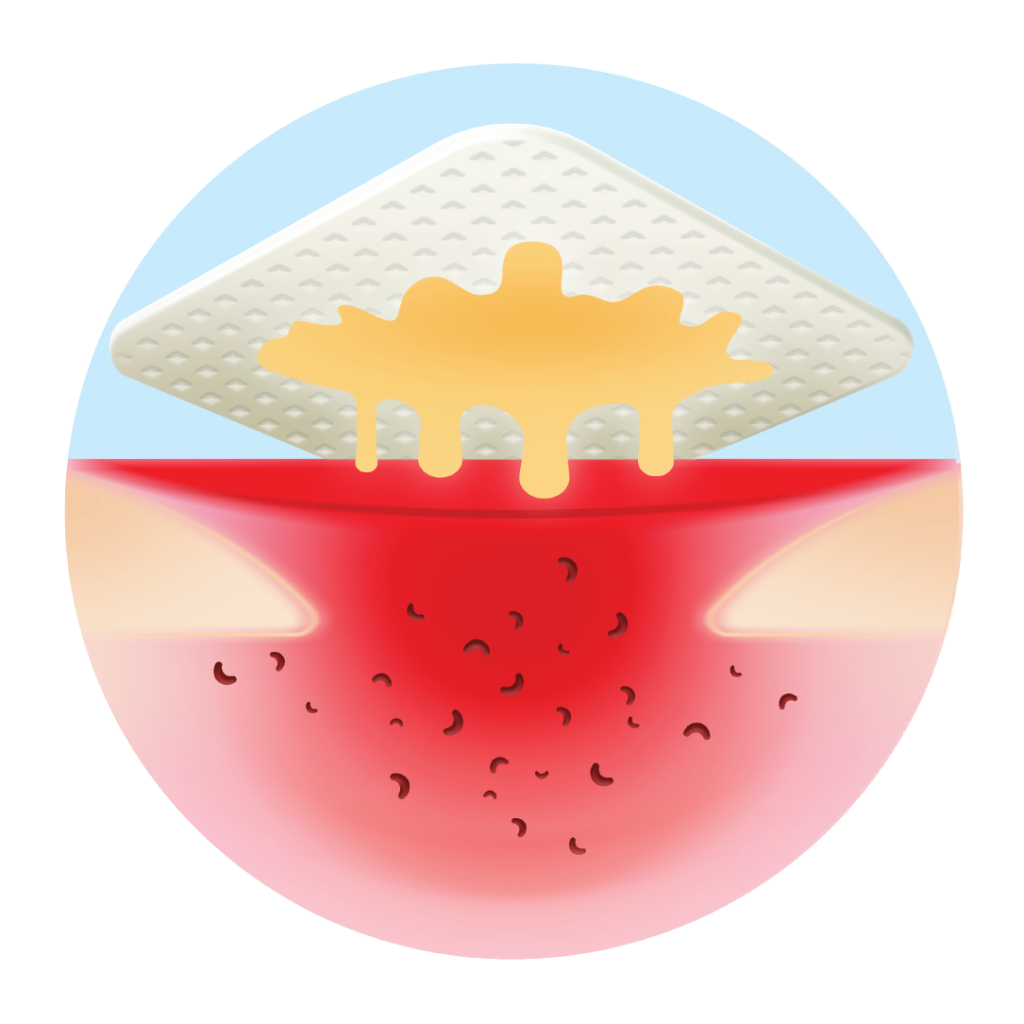
Polyabsorbent fibres trap slough and keep the wound clean
You can use 1 simple treatment on all tissue types: sloughy, granulating and epithelizing wounds

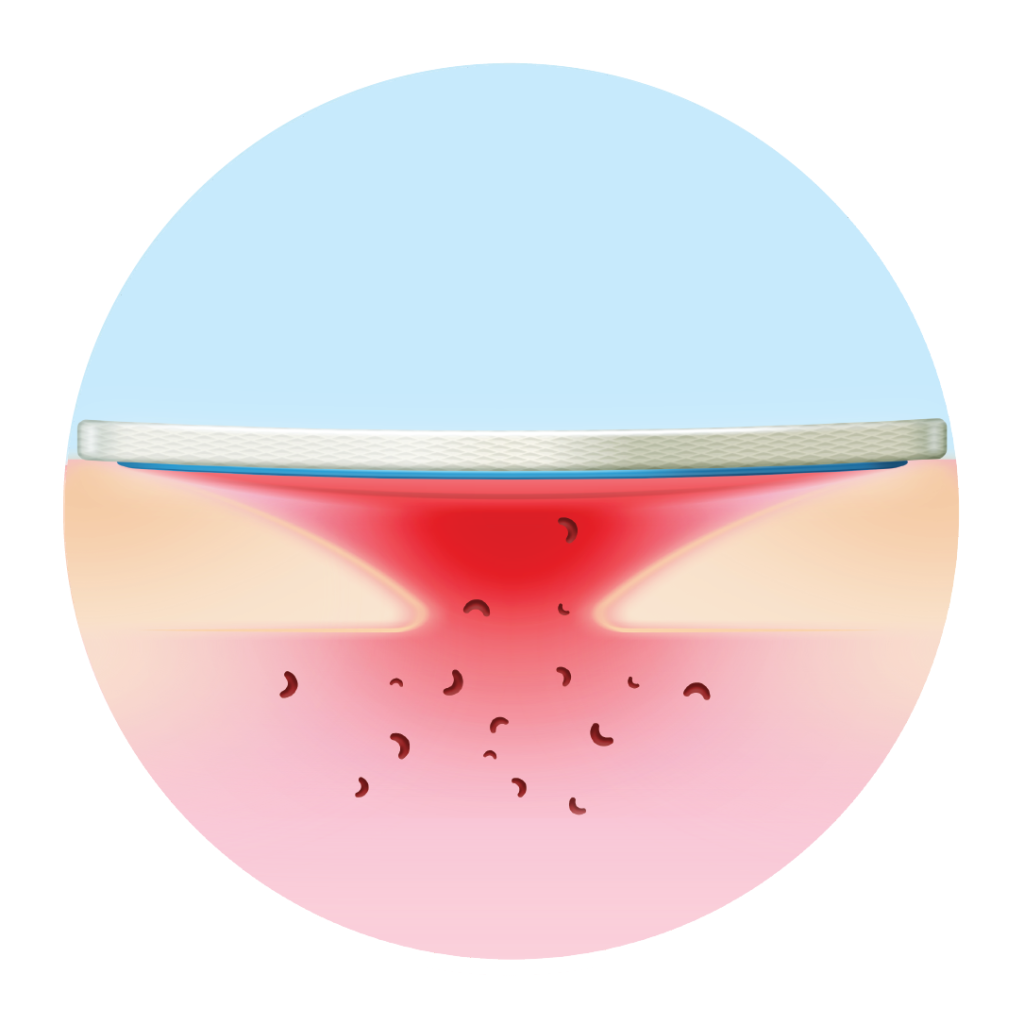
Healing matrix reduces MMP levels and restores balance
Restores balance to the patient’s healing trajectory

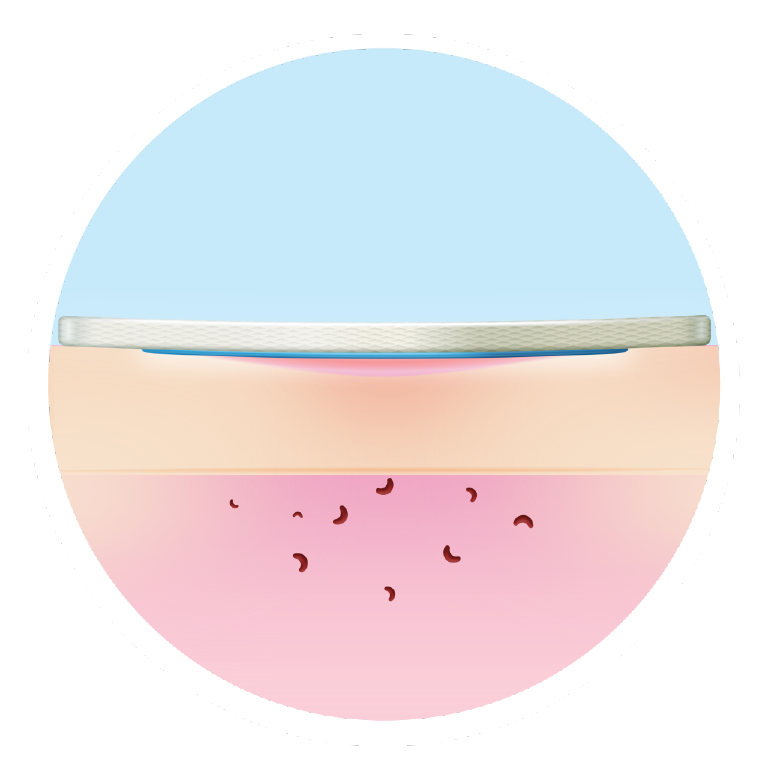
Closes wounds sooner than other dressings
The sooner you implement it, the better the outcomes5,6,7
EASY TO USE: 3 FORMATS

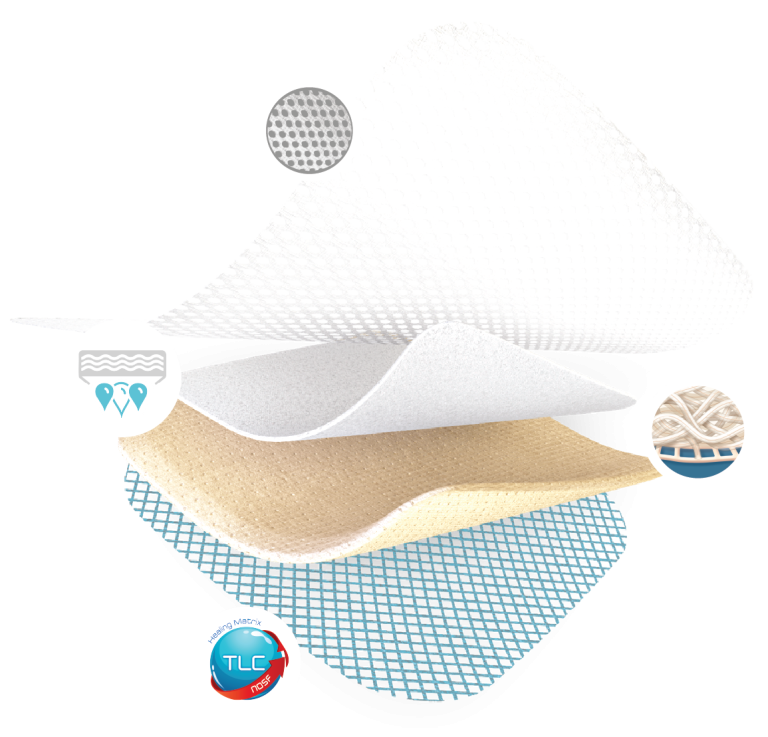
All-in-one adhesive pad

Previous
Next

Silicone backing
- Easy to apply & Ready-to-use
- Stays in place for up to 7 days
- Shower-proof
Highly absorbant layer
- Absorbs and retains exudate
Polyabsorbant fibres
- Traps slough and maintains a clean wound
- Absorbs exudate
- Can be used at all healing stages
TLC-NOSF Healing Matrix
- Heals the wound and closes it sooner
- Maintains a moist environment
- Allows a pain-free, atraumatic removal

Soft-adherent pad


Previous
Next
Polyabsorbant fibres
- Traps slough and maintains a clean wound
- Absorbs exudate
- Can be used at all healing stages
TLC-NOSF Healing Matrix
- Heals the wound and closes it sooner
- Maintains a moist environment
- Allows a pain-free, atraumatic removal

Flexible contact layer

Previous
Next
TLC-NOSF Healing Matrix
- Heals the wound and closes it sooner
- Maintains a moist environment
- Allows a pain-free, atraumatic removal
Play Video
RELIABLE: NICE RECOMMENDED
UrgoStart is recommended by NICE as a cost saving option to treat diabetic foot ulcers and venous leg ulcers. NICE recognises that this range of dressings can improve wound healing for diabetic foot ulcers and improve the rate of wound healing for venous leg ulcers. (8)
![]() Improves healing outcomes (8)
Improves healing outcomes (8)
![]() Saves money (8)
Saves money (8)
![]() Is easy to implement (9)
Is easy to implement (9)

The only dressing recommended by international guidelines to treat diabetic foot ulcers(10)
Get in touch with your local representative for more information about the UrgoStart Plus Range
References:
- Meaume S, Dissemond J, Addala A. Evaluation of two fibrous wound dressings for the management of leg ulcers: results of a European randomised controlled trial (EARTH RCT). J Wound Care 2014; 23: 3, 105–116.
- Sigal ML, Addala A, Maillard H, Chahim M, Sala F, Blaise S, Dalac S, Meaume S, Bohbot S, Tumba C, Tacca O. Clinical evaluation of a new TLC-NOSF dressing with poly-absorbent fibers for the local management of exuding leg ulcers, at the different stages of the healing process: Results from two multicentric, single-arm, prospective, open-label clinical trials. J Wound Care 2019: 28(3) :164-17
- “In vitro” study. Internal Report. Laboratoires URGO
- Edmonds M, Lázaro-Martínez JL, Alfayate-García JM, Martini J, Petit JM, Rayman G, Lobmann R, Uccioli L, Sauvadet A, Bohbot S, Kerihuel JC, Piaggesi A. Sucrose octasulfate dressing versus control dressing in patients with neuroischaemic diabetic foot ulcers (Explorer): an international, multicentre, double-blind, randomised, controlled trial. Lancet Diabetes Endocrinol. 2018 Mar;6(3):186-196.
- Münter KC, Meaume S, Augustin M, Senet P, Kérihuel J.C. The reality of routine practice: a pooled data analysis on chronic wounds treated with TLC-NOSF wound dressings. J Wound Care. 2017 Feb; 26 (Sup2): S4-S15. Erratum in: J Wound Care. 2017 Mar 2; 26(3): 153
- Lazaro et al . Optimal wound closure of diabetic foot ulcers with early initiation of TLC-NOSF treatment: post-hoc analysis of Explorer.JWC VOL 28, NO 6, June 2019
- Dissemond J, Lützkendorf S, Dietlein M, Neßeler I, Becker E, Möller U, Thomassin L, Bohbot S, Münter KC. Clinical evaluation of polyabsorbent TLC-NOSF dressings on chronic wounds: a prospective, observational, multicentre study of 1140 patients. J Wound Care. 2020 Jun 2;29(6):350-361. doi: 10.12968/jowc.2020.29.6.350. PMID: 32530781.
- National Institute for Health and Care Excellence (NICE), UrgoStart for treating leg ulcers and diabetic foot ulcers, https://www.nice.org.uk/guidance/mtg42, April 2023
- Adoption support resource – insights from the NHS, ://www.nice.org.uk/guidance/mtg42/resources/adoption-support-resource-insights-from-the-nhs-6718514509/chapter/1-Introduction, March 2019
- In the treatment of diabetic foot ulcers. IWGDF Guidelines on the prevention and management of diabetic foot disease, 2019
- Meaume S, Truchetet F, Cambazard F et al. A randomized, controlled, double-blind prospective trial with a Lipido-Colloid Technology-Nano-OligoSaccharide Factor wound dressing in the local management of venous leg ulcers. Wound Repair Regen. 2012; 20: 4, 500–511. |
- Meaume S, Dompmartin A, Lazareth I, Sigal M, Truchetet F, Sauvadet A, Bohbot S. Quality of life in patients with leg ulcers: results from CHALLENGE, a double-blind randomized controlled trial. Journal of Wound Care. 2017; 26 (7): 368-379. |
- Schmutz J.L. et al. Evaluation of the nano-oligosaccharide factor lipido-colloid matrix in the local management of venous leg ulcers : results of a randomised, controlled trial. Int Wound J 2008;5:172–182
- Augustin M, Keuthage W, Lobmann R, Lützkendorf S, Groth H, Möller U, Thomassin L, Bohbot S, Dissemond J, Blome C. Clinical evaluation of UrgoStart Plus dressings in real-life conditions: results of a prospective multicentre study on 961 patients. J Wound Care. 2021 Dec 2;30(12):966-978. doi: 10.12968/jowc.2021.30.12.966. PMID: 34881999.


