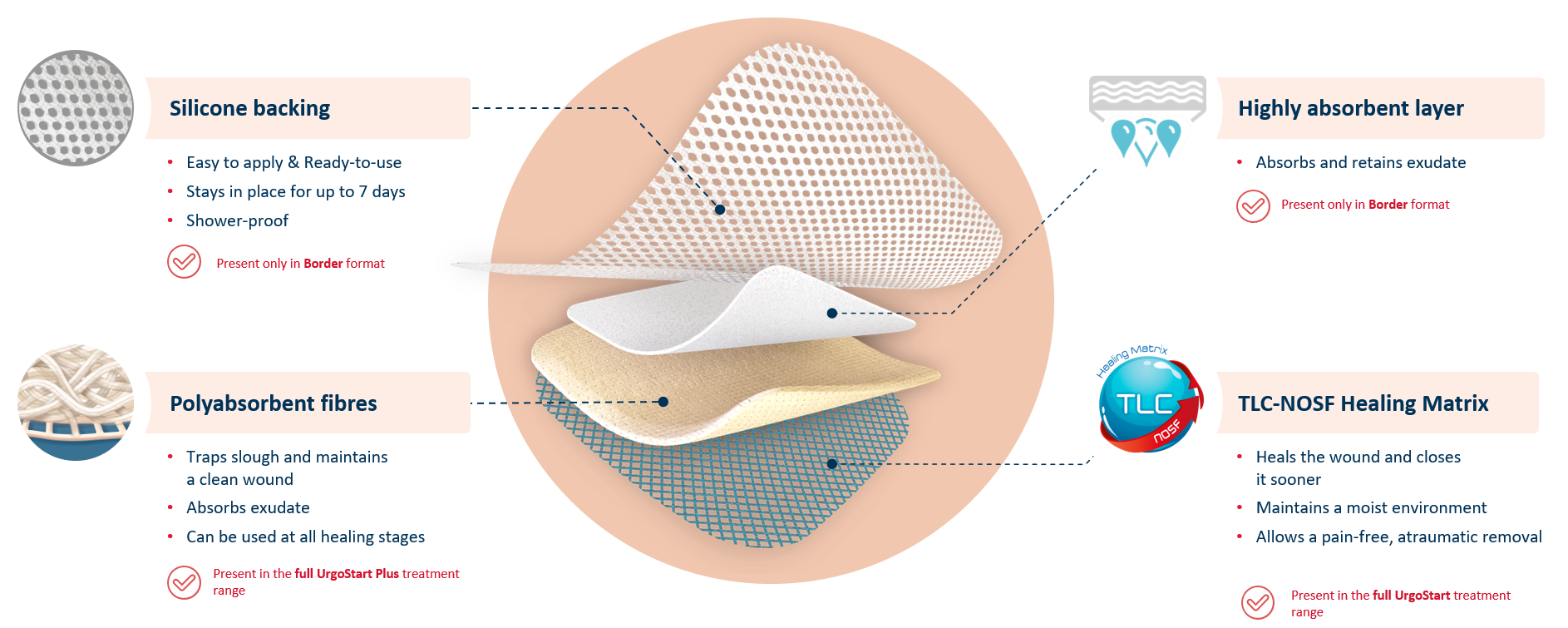
UrgoStart Plus Border is an all-in-one adhesive pad made of 4 unique layers. Thanks to its composition, UrgoStart Plus Border:
- Continuously cleans: its poly-absorbent fibres trap and retain exudate, slough and debris (1,2)
- Heals & closes the wound sooner: its TLC-NOSF** healing matrix reduces excess matrix metalloproteinases (MMPs), rebalancing the wound and closing it sooner.(2,3,4)
The efficacy of TLC-NOSF to close wounds sooner has been proven by the highest level of clinical evidence.(5,6,7) The earlier TLC-NOSF treatment is started, the more effective it is.6,7)
- Maintains a most environment optimal for healing
- Allows a gentle, pain-free removal
UrgoStart Plus Border also contains a highly absorbent layer, and silicone backing. This means that UrgoStart Plus Border can manage highly exuding wounds, while being shower-proof and and easy to apply and reposition.
Finally, UrgoStart Plus Border is always used as part of a global management approach, along with appropriate etiological treatment.
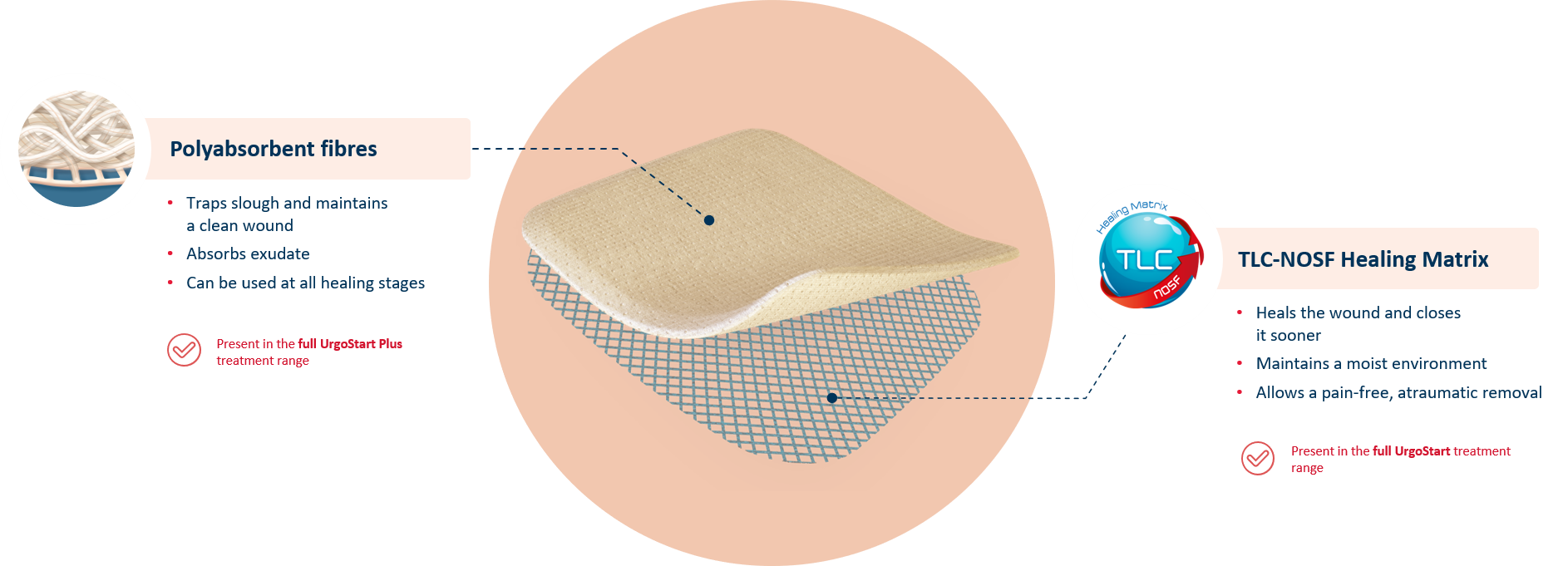
UrgoStart Plus Pad is a soft-adherent pad. Thanks to its unique composition, UrgoStart Plus Pad:
- Continuously cleans: its poly-absorbent fibres trap and retain exudate, slough and debris(1,2)
- Heals & closes the wound sooner: its TLC-NOSF** healing matrix reduces excess matrix metalloproteinases (MMPs), rebalancing the wound and closing it sooner.(2,3,4)
The efficacy of TLC-NOSF to close wounds sooner has been proven by the highest level of clinical evidence.(5,6,7) The earlier TLC-NOSF treatment is started, the more effective it is.(6,7)
- Maintains a most environment optimal for healing
- Allows a gentle, pain-free removal
UrgoStart Plus Pad is always used as part of a global management approach, along with appropriate etiological treatment.
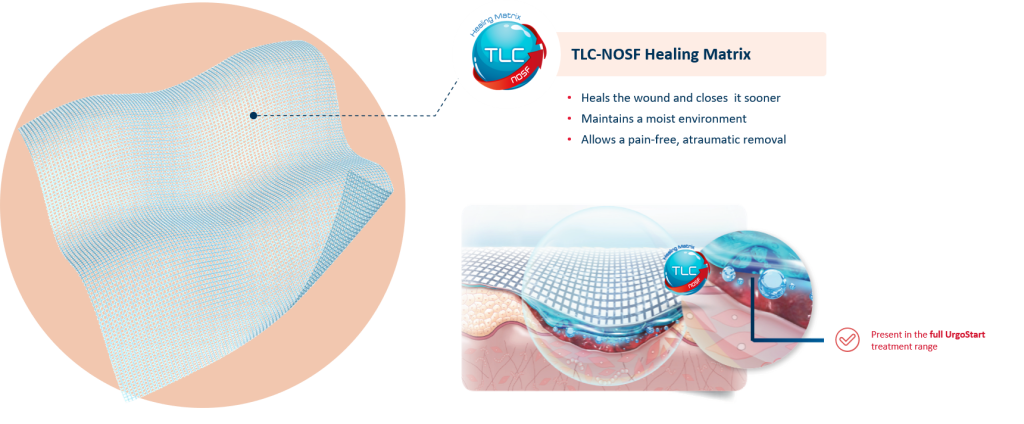
UrgoStart Contact is a non-adhesive, non-occlusive, and highly conformable contact layer impregnated with the patented TLC-NOSF** healing matrix. Thanks to its composition, UrgoStart Contact:
- Heals & closes the wound sooner: its patented TLC-NOSF** healing matrix reduces excess matrix metalloproteinases (MMPs), rebalancing the wound and closing it sooner. (2,3,4)
The efficacy of TLC-NOSF to close wounds sooner has been proven by the highest level of clinical evidence.(5,6,7) The earlier TLC-NOSF treatment is started, the more effective it is.(6,7)
- Maintains a most environment optimal for healing
- Allows a gentle, pain-free removal
UrgoStart Contact is always used as part of a global management approach, along with appropriate etiological treatment.
UrgoStart Plus Border is indicated for all wound bed stages – sloughy (0-100%), granulating and epithelialising – of moderately to heavily exuding wounds. This means it is effective at every wound healing phase*, from the start* and through to complete healing.
UrgoStart Plus Border is indicated for treating the following skin wounds:
- Leg ulcers
- Diabetic foot ulcers
- Pressure injuries
- Other wounds at risk of longlasting
Contraindications:
- Wounds with weakened periwound skin.
- Mucous membrane wounds.
- Malignant wounds.
- Fistula wounds.
- Epidermolysis bullosa wounds.
- Necrotic wounds (wounds covered with black dead tissue, eschars).
- Haemorrhagic wounds: UrgoStart Plus Border can be used to help stop minor bleeding but should not be used as a surgical sponge.
- Known hypersensitivity of the patient to one of the TLC or TLC-NOSF dressings, or to carboxymethylcellulose (CMC).
UrgoStart Plus Pad is indicated for all wound bed stages – sloughy (0-100%), granulating and epithelialising – of low to moderately exuding wounds. This means it is effective at every wound healing phase*, from the start* and through to complete healing.
UrgoStart Plus Pad is indicated for treating the following skin wounds:
- Leg ulcers
- Diabetic foot ulcers
- Pressure injuries
- Other wounds at risk of longlasting
UrgoStart Plus Pad can be used underneath any secondary dressing. For highly exuding wounds, use UrgoStart Plus Pad in combination with a superabsorber.
Contraindications:
- Mucous membrane wounds.
- Malignant wounds.
- Fistula wounds.
- Epidermolysis bullosa wounds.
- Necrotic wounds (wounds covered with black dead tissue, eschars).
- Haemorrhagic wounds: UrgoStart Plus Pad can be used to help stop minor bleeding but should not be used as a surgical sponge.
- Known hypersensitivity of the patient to one of the TLC or TLC-NOSF dressings, or to carboxymethylcellulose (CMC).
UrgoStart Contact is indicated for treating the following skin wounds:
- Leg ulcers
- Diabetic foot ulcers
- Pressure injuries
- Other wounds at risk of longlasting
UrgoStart Contact is suitable for granulating and epithelialising wounds, especially those in difficult-to-dress locations.
UrgoStart Contact can be used underneath any secondary dressing. For highly exuding wounds, use UrgoStart Contact in combination with a superabsorber.
Contraindications:
- • Malignant wounds
• Fistula wounds
• Wounds on mucous membranes
• Known hypersensitivity of the patient to one of the TLC or TLC-NOSF dressings, or to
carboxymethylcellulose (CMC).
.

Before use
- Wash your hands with soap and water and dry them with a clean towel; or disinfect your hands with a hydro-alcoholic solution or gel.
- Put on disposable medical gloves.
- Prepare the wound according to the usual wound management protocol. When debridement is deemed necessary by the caregiver, the use of UrgoStart Plus Border does not exempt it. The wound may appear larger after being cleaned or after debridement . Gently dry the skin around the wound with a sterile pad.
- Remove the disposable medical gloves.
- Check that the pouch that maintains the sterile condition of UrgoStart Plus Border has not been damaged or unintentionally opened before use. If this is the case, use another one.
To apply UrgoStart Plus Border
- Open the UrgoStart Plus Border pouch on its full length, without touching the dressing. Do nottake UrgoStart Plus Border out of its pouch at this stage.
- Put on new disposable medical gloves.
- Take UrgoStart Plus Border out of the opened pouch, avoiding touching the outside of the pouch.
- UrgoStart Plus Border consists of several absorbent layers that must not be cut. Only the adhesive border can be cut with sterile scissors where it can facilitate application and dressing securement.
- In order to facilitate handling, remove one of the two protective tabs from UrgoStart Plus Border, start applying the dressing from its part released from the protection, and remove the second protective tab to fully apply the dressing.
- Be careful to place the adhesive border at least one centimetre (1 cm) from the wound edges.
Sacrum format: place the tip of the dressing towards the bottom of the sacrum area (at thestart of the intergluteal cleft).
Replacement frequency and treatment duration
The replacement frequency is left to the discretion of the healthcare professional. However, it is
recommended to replace UrgoStart Plus Border every 1 to 2 days for wounds with fibrin (yellowish
slough on the wound bed) and then to adapt the replacement frequency according to the clinical assessment of the wound and the level of exudate (that is, the amount of fluid in the wound) without exceeding 7 days of use per dressing. It is recommended to continue treatment with UrgoStart Plus Border until complete wound healing. The time to complete healing depends on the characteristics of the wound (for example, its size, duration, and recurrence) as well as on the patient (for example, age, mobility and comorbidities), and can exceed 8 weeks.
Precautions for use
In the event of signs or symptoms of infection before or after starting treatment with UrgoStart Plus Border, such as erythema (redness of the skin around the wound), oedema (swelling), heat or pain in the wound area, fever, enlargement of the wound, odour from the wound, or profuse exudate (that is, a large amount of fluid in the wound), consult a healthcare professional for appropriate treatment. In the event of overgranulation (excessive raised formation of red budding tissue on the wound) before or after starting treatment with UrgoStart Plus Border, it must be reported to a healthcare professional for appropriate care.
Before use
- Wash your hands with soap and water and dry them with a clean towel, or disinfect your hands with a hydro-alcoholic solution or gel.
- Put on disposable medical gloves.
- Prepare the wound according to the usual wound management protocol. When debridement is deemed necessary by the caregiver, the use of UrgoStart Plus Pad does not exempt it. The wound may appear larger after being cleaned or after debridement. Gently dry the skin around the wound with a sterile pad.
- Remove the disposable medical gloves.
- Check that the pouch that maintains the sterile condition of UrgoStart Plus Pad has not been damaged or unintentionally opened before use. If this is the case, use another one.
- Provide a suitable securement system (for example, medical tape, flat or tubular bandage). For a highly exuding wound (that is, a wound with a high amount of fluid), provide an additional suitable absorbent dressing. If this latter is not adhesive, provide a suitable securement system (for example, medical tape, flat or tubular bandage).
To apply UrgoStart Plus Pad
- Open the UrgoStart Plus Pad pouch on its full length, without touching the dressing. Do not take UrgoStart Plus Pad out of its pouch at this stage.
- Put on new disposable medical gloves.
- Take UrgoStart Plus Pad out of the opened pouch, avoiding touching the outside of the pouch.
- UrgoStart Plus Pad can be cut with sterile scissors where it can facilitate application and dressing securement.
- In order to facilitate handling, remove one of the two protective tabs from UrgoStart Plus Pad, start applying the dressing from its part released from the protection, and remove the second protective tab to fully apply the dressing (this helps prevent the dressing from adhering to the gloves).
- For a highly exuding wound (that is, a wound with a high amount of fluid), cover UrgoStart Plus Pad with the previously prepared additional absorbent dressing.
- Apply the securement system previously prepared (for example, medical tape, flat, tubular mesh, or adhesive dressing).
Replacement frequency and treatment duration
The replacement frequency is left to the discretion of the healthcare professional. However, it is recommended to replace UrgoStart Plus Pad every 1 to 2 days for wounds with fibrin (yellowish slough on the wound bed) and then to adapt the replacement frequency according to the clinical assessment of the wound and the level of exudate (that is, the amount of fluid in the wound) without exceeding 7 days of use per dressing. If is recommended to continue treatment with UrgoStart Plus Pad until complete wound healing. The time to complete healing depends on the characteristics of the wound (for example, its size, duration, and recurrence) as well as on the patient (for example, age, mobility and comorbidities) and can exceed 8 weeks.
Precautions for use
In the event of signs or symptoms of infection before or after starting treatment with UrgoStart Plus Pad, such as erythema (redness of the skin around the wound), oedema (swelling), heat or pain in the wound area, fever, enlargement of the wound, odour from the wound, or profuse exudate (that is, a large amount of fluid in the wound), consult a healthcare professional for appropriate treatment. In the event of overgranulation (excessive raised formation of red budding tissue on the wound) before or after starting treatment with UrgoStart Plus Pad, it must be reported to a healthcare professional for appropriate care.
Before use
- Wash your hands with soap and water and dry them with a clean towel, or disinfect your hands with a hydro-alcoholic solution or gel.
- Put on disposable medical gloves.
- Prepare the wound according to the usual wound management protocol. Gently dry the skin around the wound with a sterile pad.
- Remove the disposable medical gloves.
- Check that the pouch that maintains the sterile condition of UrgoStart Contact has not been damaged or unintentionally opened before use. If this is the case, use another one.
- Provide an absorbent dressing suitable to the level of exudate (that is, the amount of fluid appearing at the wound). If this latter is not adhesive, provide an appropriate securement system too (for example, medical tape, flat or tubular bandage).
To apply UrgoStart Contact
- Open the UrgoStart Contact pouch on its full length, without touching the dressing. Do not take UrgoStart Contact out of its pouch at this stage.
- Put on new disposable medical gloves. UrgoStart Contact adheres to latex gloves. Moistening the latex gloves with water or physiological saline may facilitate handling.
- Take UrgoStart Contact out of the opened pouch, avoiding touching the pouch.
- UrgoStart Contact can be cut with sterile scissors when this can facilitate its application.
- Remove the 2 protective films from UrgoStart Contact
- Apply UrgoStart Contact to the wound. For cavity or deep wounds or for wicking a deep wound, ensure that part of UrgoStart Contact is visible outside the wound, to facilitate removal when it will be replaced.
- Cover UrgoStart Contact with the previously prepared absorbent dressing.
- If the absorbent dressing is not adhesive, apply the securement system previously prepared.
- Apply a compression system when compression therapy is prescribed. When appropriate, set up the foot offloading system.
Replacement frequency and treatment duration
It is recommended to replace UrgoStart Contact every 2 to 4 days, even every 7 days, according to the clinical assessment of the wound and the level of exudate (that is, the amount of fluid appearing at the wound). In any case, the replacement frequency is left to the discretion of the healthcare professional. It is recommended to continue treatment with UrgoStart Contact until complete wound healing. The time to complete healing depends on the characteristics of the wound (for example, its size, age and recurrence) as well as on the patient (for example, age, mobility and co-morbidities), and can exceed 8 weeks.
Precautions
UrgoStart Contact is not intended for use on fully fibrinous wounds (wounds completely covered with yellowish tissue) or in the presence of necrosis (black tissue in the wound). In the event of signs or symptoms of infection before starting or during using
UrgoStart Contact such as erythema (redness of the skin around the wound), oedema (swelling), heat or pain in the wound area, fever, enlargement of the wound, odour from the wound, profuse exudate (that is, fluid appearing at the wound in large quantity), consult a healthcare professional for appropriate treatment. In the event of over-granulation (excessive formation of red tissue in the wound) before or during using UrgoStart Contact, this must be reported to a healthcare professional in order to be resolved. In the absence of clinical data, Urgostart Contact is not recommended for EB wounds
 In 2012, the Journal of Wound Care published the CHALLENGE(8) study, demonstrating that TLC-NOSF treatment leads to a greater reduction in wound surface area compared to a neutral dressing.
In 2012, the Journal of Wound Care published the CHALLENGE(8) study, demonstrating that TLC-NOSF treatment leads to a greater reduction in wound surface area compared to a neutral dressing. In December 2018, The Lancet Diabetes & Endocrinology international medical journal published the EXPLORER(4) clinical trial, demonstrating that TLC-NOSF treatment heals 60% more patients compared to a neutral dressing and that the earlier TLC-NOSF treatment is started, the more effective it is(2).
In December 2018, The Lancet Diabetes & Endocrinology international medical journal published the EXPLORER(4) clinical trial, demonstrating that TLC-NOSF treatment heals 60% more patients compared to a neutral dressing and that the earlier TLC-NOSF treatment is started, the more effective it is(2). The REALITY(7) analysis, published in 2017 in the Journal of Wound Care, presents a compilation of eight observational studies conducted in more than 10,000 patients and shows that TLC-NOSF reduces the healing time of chronic wounds by an average of 100 days and that the earlier TLC-NOSF treatment is started, the more effective it is.
The REALITY(7) analysis, published in 2017 in the Journal of Wound Care, presents a compilation of eight observational studies conducted in more than 10,000 patients and shows that TLC-NOSF reduces the healing time of chronic wounds by an average of 100 days and that the earlier TLC-NOSF treatment is started, the more effective it is.
In 2020, the Journal of Wound Care published the last URGOSTART PLUS OBSERVATIONAL STUDY conducted in 1,140 patients, demonstrating that UrgoStart Plus is effective irrespective of the wound type, whatever the wound phase and however long it has been present(9).
 The HAS (French National Authority for Health) has granted the entire range a level III clinical added value (CAV)(10) something that is unprecedented for a dressing.
The HAS (French National Authority for Health) has granted the entire range a level III clinical added value (CAV)(10) something that is unprecedented for a dressing. The URGO Group was awarded the 2018 Galien France medical device prize for UrgoStart®. Each year, this prestigious award recognises exceptional innovations in the field of health.
The URGO Group was awarded the 2018 Galien France medical device prize for UrgoStart®. Each year, this prestigious award recognises exceptional innovations in the field of health. In January 2019, and reviewed in April 2023, the United Kingdom’s NICE (National Institute for Health and Care Excellence) recommended TLC-NOSF treatment for the wound care of diabetic foot ulcers and venous leg ulcers(11). The NICE recommendations support the fact that the UrgoStart® range has better results in terms of reducing wound healing time, improving patients’ quality of life and enabling significant savings for health authorities compared to neutral dressings.
In January 2019, and reviewed in April 2023, the United Kingdom’s NICE (National Institute for Health and Care Excellence) recommended TLC-NOSF treatment for the wound care of diabetic foot ulcers and venous leg ulcers(11). The NICE recommendations support the fact that the UrgoStart® range has better results in terms of reducing wound healing time, improving patients’ quality of life and enabling significant savings for health authorities compared to neutral dressings. 2019: IWGDF (International Working Group on the Diabetic Foot) guidelines This is the very first time that a dressing has been recommended by the IWGDF.(12)
2019: IWGDF (International Working Group on the Diabetic Foot) guidelines This is the very first time that a dressing has been recommended by the IWGDF.(12)Get in touch with your local representative for more information about the UrgoStart Plus Range
UrgoStart: Urgo Medical | UrgoStart reduces ulcer healing time
References:
*Excluding dry necrosis.
**TLC-NOSF: Lipido-Colloid technology – Nano OligaSaccharide Factor (KSOS: Potassium octasulfate sucrose salt).
- Meaume S, Dissemond J, Addala A. Evaluation of two fibrous wound dressings for the management of leg ulcers: results of a European randomised controlled trial (EARTH RCT). J Wound Care 2014; 23: 3, 105–116.
- Sigal ML, Addala A, Maillard H, Chahim M, Sala F, Blaise S, Dalac S, Meaume S, Bohbot S, Tumba C, Tacca O. Clinical evaluation of a new TLC-NOSF dressing with poly-absorbent fibers for the local management of exuding leg ulcers, at the different stages of the healing process: Results from two multicentric, single-arm, prospective, open-label clinical trials. J Wound Care 2019: 28(3) :164-17
- “In vitro” study. Internal Report. Laboratoires URGO
- Edmonds M, Lázaro-Martínez JL, Alfayate-García JM, Martini J, Petit JM, Rayman G, Lobmann R, Uccioli L, Sauvadet A, Bohbot S, Kerihuel JC, Piaggesi A. Sucrose octasulfate dressing versus control dressing in patients with neuroischaemic diabetic foot ulcers (Explorer): an international, multicentre, double-blind, randomised, controlled trial. Lancet Diabetes Endocrinol. 2018 Mar;6(3):186-196.
- Edmonds M et al. Sucrose octasulfate dressing versus control dressing in patients with neuroischaemic diabetic foot ulcers (Explorer): an international, multicentre, double-blind, randomised, controlled trial. Lancet Diabetes Endocrinol. 2018 Mar; 6(3): 186-196.
- Lázaro-Martínez JL et al. Optimal wound closure of diabetic foot ulcers with early initiation of TLC-NOSF treatment: post-hoc analysis of Explorer. J Wound Care. 2019 Jun 2; 28(6): 358-367.
- Münter KC et al. The reality of routine practice: a pooled data analysis on chronic wounds treated with TLC-NOSF wound dressings. J Wound Care. 2017 Feb; 26 (Sup2): S4-S15. Erratum in: J Wound Care. 2017 Mar 2; 26(3): 153.
- Meaume S, Truchetet F, Cambazard F et al. A randomized, controlled, double-blind prospective trial with a Lipido-Colloid Technology-Nano-OligoSaccharide Factor wound dressing in the local management of venous leg ulcers. Wound Repair Regen. 2012; 20: 4, 500–511.
- Dissemond J, Lützkendorf S, Dietlein M, Neßeler I, Becker E, Möller U, Thomassin L, Bohbot S, Münter KC. Clinical evaluation of polyabsorbent TLC-NOSF dressings on chronic wounds: a prospective, observational, multicentre study of 1140 patients. J Wound Care. 2020 Jun 2;29(6):350-361. doi: 10.12968/jowc.2020.29.6.350. PMID: 32530781
- CAV lll: in non-infected (IDSA/IWGDF infection criteria) neuro-ischaemic diabetic foot ulcers (non-critical ischaemia, in the granulation phase (sequential treatment
- National Institute for Health and Care Excellence (NICE), UrgoStart for treating leg ulcers and diabetic foot ulcers, https://www.nice.org.uk/guidance/mtg42, April 2023
- IWGDF – Guidelines on the prevention and management of diabetic foot disease – 2019

