UrgoTul Range
Page updated on 15 January 2024
Homepage » Urgo solutions » UrgoTul Range
The UrgoTul Family range of dressings are for patients whose skin needs that extra TLC. The Technology Lipido-Colloid (TLC) healing matrix:
Enables key cells for healing (4,5)


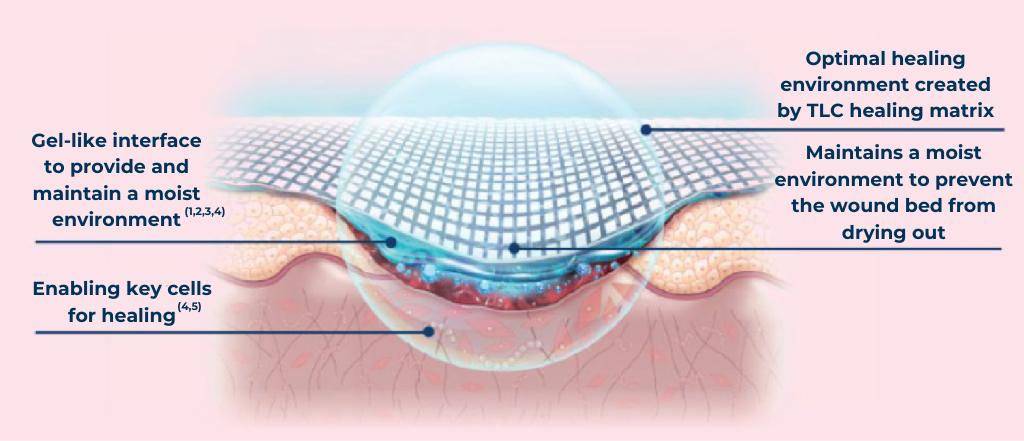
Mode of action where the TLC (Technology Lipido-Colloid) Healing Matrix is in contact with the wound
Specifically designed to improve clinical outcomes
![]() Provides and maintains a moist wound healing environment(1,2,3,4)
Provides and maintains a moist wound healing environment(1,2,3,4)
![]() Stimulates fibroblast proliferation(4,5)
Stimulates fibroblast proliferation(4,5)
![]() Pain-free and atraumatic removal(6)
Pain-free and atraumatic removal(6)
![]() Clinically evaluated on over 54,000 patients in observational and clinical studies(7)
Clinically evaluated on over 54,000 patients in observational and clinical studies(7)


TLC Healing Matrix



Absorbent polyurethane foam
TLC Healing Matrix

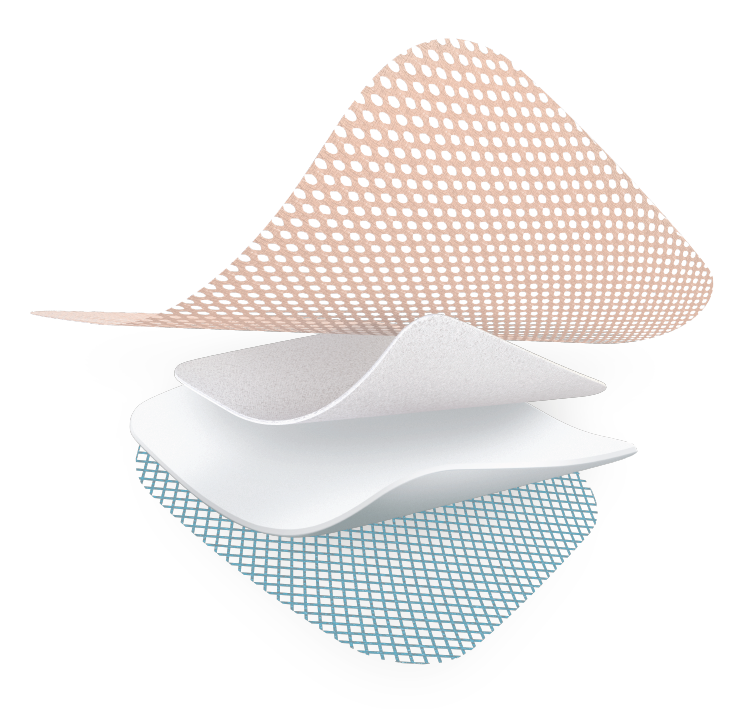


Silicone backing
Highly absorbant layer
Absorbent polyurethane foam
TLC Healing Matrix

1. Le Berre M, Lurton Y, Maia S, Roebroeck V, Durand J, Gicquel V, Basle B. Pansements impregnes : tulles/interfaces. Poster, CPC 2005, Paris.
2. Parpex P. et al. Management of venous leg ulcers with Cellosorb Micro-adherent dressing: results of a multi-centre clinical trial. Phlebologie 2010; 63: 76-82.
3. Meaume S, et al. Use of a new, flexible lipidocolloid dressing on acute and chronic wounds: results of a clinical study. J Wound Care. 2011;20(4):180,182-5.
4. Bernard., F.X., Barrault, C., Juchaux, F., et al.Stimulation of the proliferation of human dermal fibroblasts in vitro by a lipidocolloid dressing. J Wound Care 2005; 14: 5, 215–220. (Study conducted on UrgoTul)
5. FX. Bernard, F. Juchaux et al., Effets d’un pansement lipido colloide sur la production de matrice extracellulaire. Journal des Plaies et gCicatrisations, 2007. (Study conducted on Urgotul).
6. Meaume, S., Teot, L., Lazareth, I. et al. The importance of pain reduction through dressing selection in routine wound management: the MAPP study. J Wound Care 2004; 13: 10, 409–413.
7. White, R., Cowan, T., Glover, D. Supporting evidence-based practice: a clinical review of TLC healing matrix (2nd edition). MA Healthcare Ltd, London, 2015.
8. Benbow M., Iosson, G. A clinical evaluation of UrgoTul to treat acute and chronic wounds. Br J Nurs 2004; 13: 2, 105–109.
9. Burton, F. An evaluation of non-adherent wound-contact layers for acute and surgical wounds J Wound Care 2004; 13: 9, 371–373.
